Magnetic Hyperthermia
Magnetic hyperthermia mediated customizable drug release
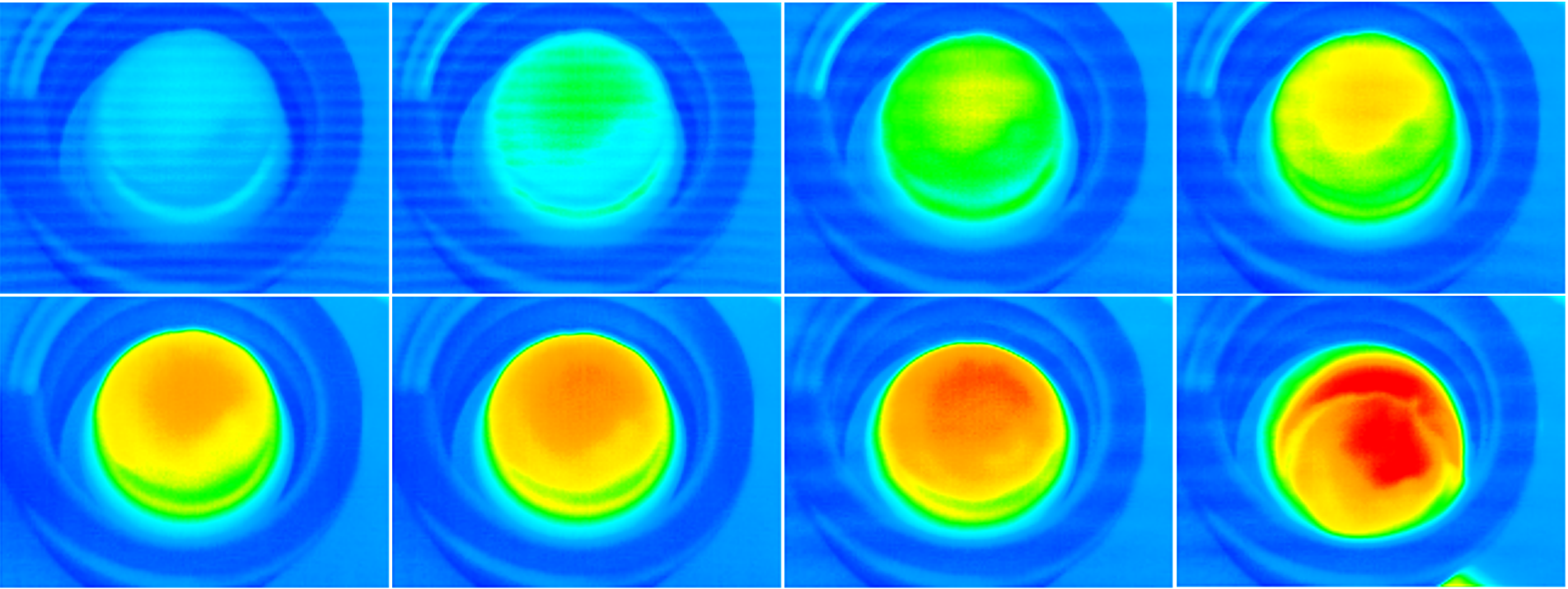
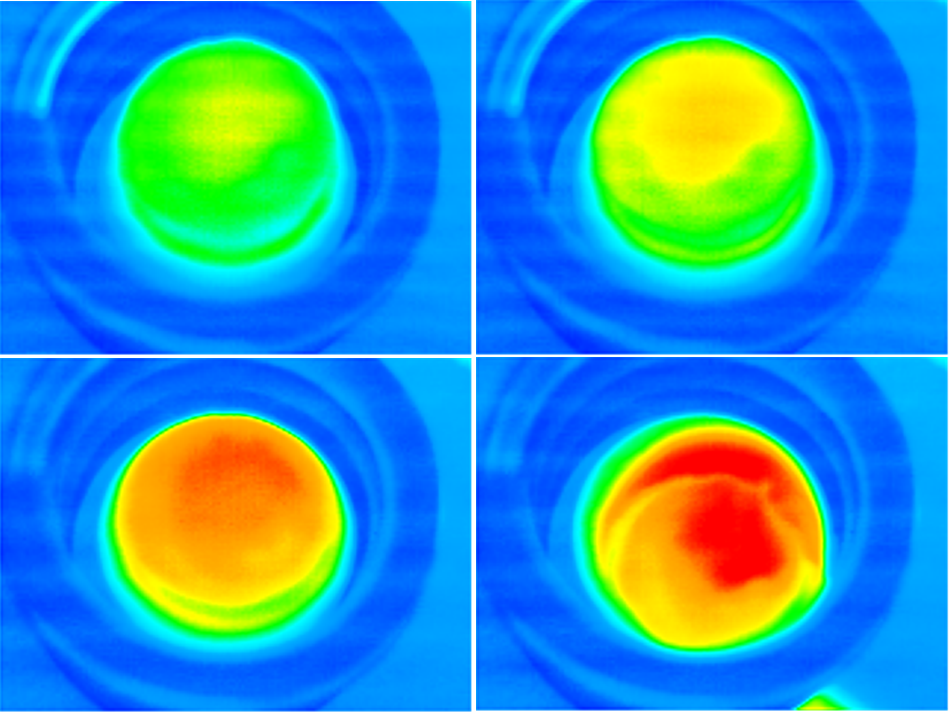
This state-of-the-art study will utilize magnetic hyperthermia, via use of iron oxide nanoparticles incorporated within hydrogel matrices to achieve shape-selective controlled drug release. Magnetic hyperthermia has shown to be highly effective in treatment of diseases such as cancer. It utilizes the ability of MNPs to transform electromagnetic energy from an external high-frequency field to heat, within a selected site (i.e., tumours). When subjected to an external oscillating magnetic field, the particles begin to vibrate and heat up their surroundings. This is effective, as cells tend to undergo necrosis at 40-43°C. The advantage to such a treatment in comparison to conventional cancer treatments such as radiotherapy and chemotherapy is that this treatment is localised. Studies also suggest that the constant tuning of such a magnetic field leads to limited thermal shock to the surround tissue outside of the targeted area. To this point there is even potential to treat tumours which are located at more sensitive areas of the body, such as spinal columns.
Iron oxide, specifically Magnetite (Fe3O4), is a proven biocompatible compound which when produced as a nanoparticles, exhibit superparamagnetism. This is incredibly useful for generating hyperthermia as the particles require only small magnetic fields to achieve the effects it was intended for. Different sizes of magnetite particles show varying effectiveness of hyperthermia, as a larger particle will exhibit less magnetic force in comparison to a smaller particle when subjected to an external magnetic field. This allows for selective fine-tuning of treatments. When applied to actuation, this allows for the controlled release of a drug into a system allowing for the differing dispersal rates and varying dosages. Thus, a combinatorial approach utilizing both techniques has the potential to be applied as a multimodal form of drug delivery, which can be tailored to suit patient needs without significantly increasing the cost of such a treatment.
Recently, the Group has developed a novel, hyperthermia-mediated shape-selective drug release methodology based on magnetic cryogels, which is highly tuneable, low-cost and safe. In conjunction with the magnetic cryogels developed within the Group, this cutting-edge technology has potential for development of new drug delivery platforms, for efficient, safe and non-invasive treatment of external wounds and cancer treatment via hyperthermia. The magnetic-polymer gels are biocompatible and their chemical-mechanical properties can be tailored to accomplish stability and robustness. The customizable properties together with biocompatibility will allow these materials to be used as wearable devices (such as gel patches), capable of drug-release, for both external and sub-epidermal applications. The magnetic component renders remote control actuation as well as the possibility to be activated via hyperthermia. Such a platform can potentially lead to significant improvements in targeted drug delivery, with minimally invasive procedures.
References:
Ayomi S. Perera, Siqi Zhang, Shervanthi Homer-Vanniasinkam, Marc-Olivier Coppens and Mohan Edirisinghe, “Polymer-magnetic composite fibers for remote-controlled drug release”, ACS Appl. Mater. Interfaces 2018, 10, 15524-15531.
Research concept: Schematic representation of the fabrication of magnetic cryogels and their application in hyperthermia-mediated drug release.
Research poster by PhD student Reece Bristow: Functionalisation of iron(II, III) oxide nanoparticles, their incorporation into polymer cryogels and their application in hyperthermia-mediated drug release .

