Cryogels
Magnetic cryogels for biomedical applications: Wound care and hyperthermia induced drug delivery
Polymer hydrogels incorporated with magnetic nanoparticles (MNPs) have vast potential in biomedical applications such as treatment of cancer and cardiovascular diseases and in regenerative medicine. The advantages of having MNPs in such platforms are that they can be remotely triggered by various methods such as hyperthermia, pH, and chemical changes; to release drugs or other desired molecules. Actuation of a drug-carrying platform by an external magnetic field (i.e., magnetic actuation), is another such possible pathway to trigger drug release in a controlled manner, along with magnetic-hyperthermia, and will be the bedrock techniques that will be explored, during the proposed PhD study. Although both methods have been researched (magnetic hyperthermia being the most prominent), a combinatorial approach that uses both magnetic actuation, in tandem with or complimentary to hyperthermia is yet to be realised, and will be attempted during the proposed study.
Such a technology will have enormous potential commercial applications, for example in the development of responsive plasters and bandages, capable of being ‘activated’ by a magnet to release drugs for the treatment of wounds. Thus, instead of passive diffusion, the drugs can be actively released with varying dosages and frequency, depending on the nature of the wound. This will essentially provide a method to clean and disinfect wounds, without the aid of a physician or changing dressings.
This dynamic interdisciplinary project will focus on developing facile methods to functionalize iron(II, III) oxide (Fe3O4) magnetic nanoparticles (MNPs), targeting applications in wound care and cancer treatment via hyperthermia. Key experiments will target the use of citric acid and dopamine as anchors to bind drug molecules to MNPs. Paracetamol and Bismuth(III) subsalicylate will be used as model drugs during this study. The MNP-anchor-drug systems will then be incorporated into polymeric hydrogels, and subject to external stimuli such as magnetic fields and heat, to trigger the release of drugs, remotely, in a controlled manner. This will lead to the development of ‘smart’ gel patches infused with known drug quantities, that can be ‘activated’ when necessary.
This cutting-edge technology has potential for development of new drug delivery platforms, for efficient, safe and non-invasive treatment of external wounds and cancer treatment via hyperthermia. The magnetic-polymer gels are biocompatible and their chemical-mechanical properties can be tailored to accomplish stability and robustness. The customizable properties together with biocompatibility will allow these materials to be used as wearable devices (such as gel patches), capable of drug-release, for both external and sub-epidermal applications. The magnetic component renders remote control actuation as well as the possibility to be activated via hyperthermia. Such a platform can potentially lead to significant improvements in targeted drug delivery, with minimally invasive procedures.
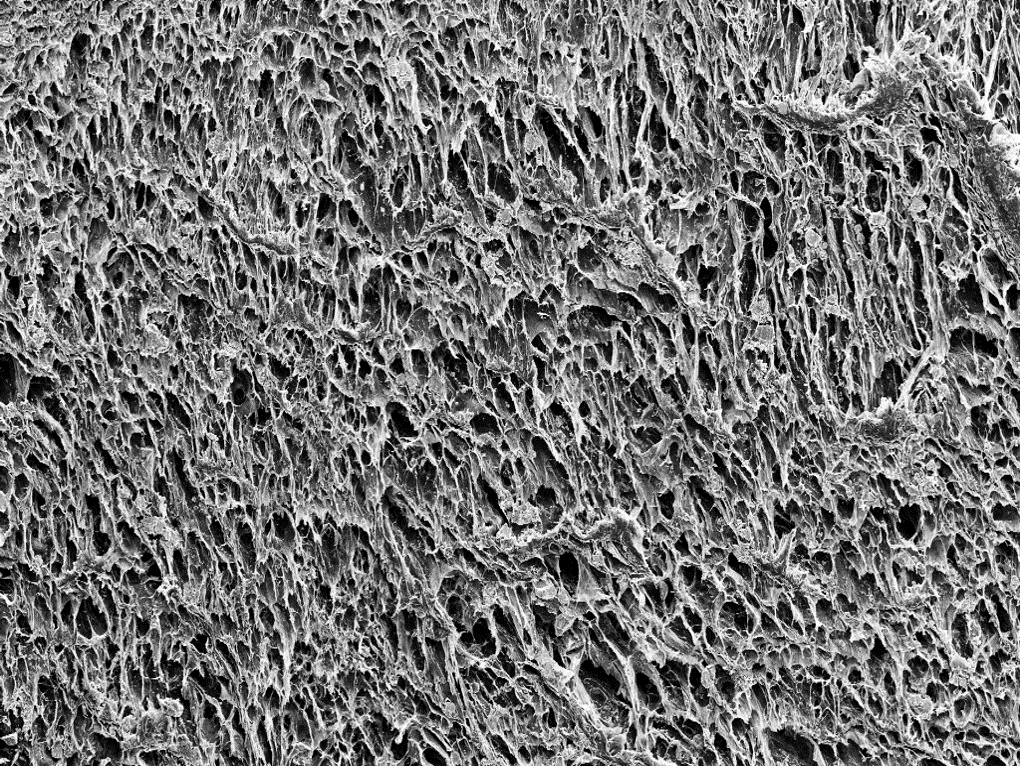
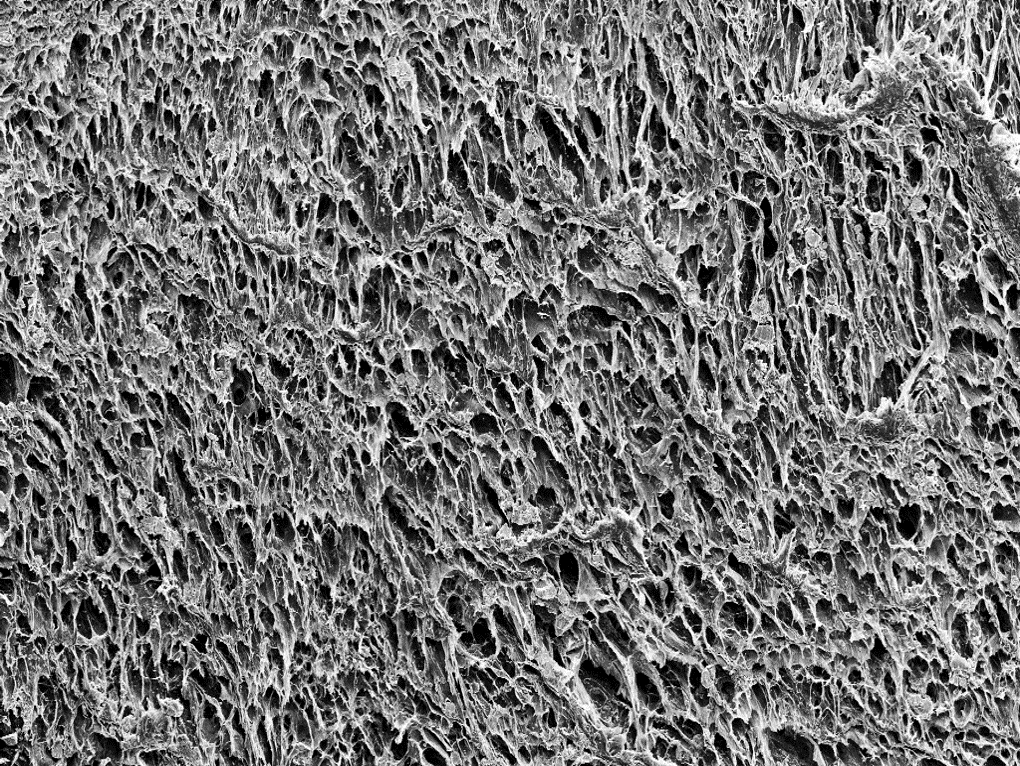
Morphology of magnetic cryogels. Left and centre: SEM images of freeze-dried drug coated PVA-MNP hydrogels. Right: EDX data for SEM image in centre, where drug coated MNP distribution is depicted in red, indicating a homogeneous distribution of the MNP-CA-Ac system within the hydrogel.
Furthermore, the biocompatibility of the gels will be evaluated as part of the interdisciplinary collaborations established with the Hill Group at the University of Plymouth. For example, drugs promoting fibroblast proliferation to enhance wound healing would be tested using 3D biomimetic ‘tissues’, in which cells are embedded within a compressed collagen matrix. Similarly, the release of chemotherapeutic drugs would be tested by incorporating cancer organoids into the matrix and evaluating cell viability and proliferation. A similar approach will be pursued in relation to microbial infection models where the destruction of polymicrobial biofilms will be assessed following the application of hydrogel matrix containing appropriate antimicrobials to help disintegrate an established biofilm community.
References:
Ayomi S. Perera, Siqi Zhang, Shervanthi Homer-Vanniasinkam, Marc-Olivier Coppens and Mohan Edirisinghe, “Polymer-magnetic composite fibers for remote-controlled drug release”, ACS Appl. Mater. Interfaces 2018, 10, 15524-15531.
Research concept: Schematic representation of the fabrication of magnetic cryogels and their application in hyperthermia-mediated drug release.
Research poster by PhD student Reece Bristow: Functionalisation of iron(II, III) oxide nanoparticles, their incorporation into polymer cryogels and their application in hyperthermia-mediated drug release .


